More than scratching the surface: Students test durability of antimicrobial materials

(From left) Matthew Williams, professor Jesse Kern, and Leo Galopin experiment with lab testing equipment
If you’ve recently visited a public place such as a gym, an airport, or a doctor’s office, you might have noticed a rough texture applied to the door knobs, toilet handles, and other frequently touched surfaces. The special material is called an antimicrobial surface, and many businesses are now incorporating the self-cleaning technology to help prevent the spread of germs.
For Summer Research, a group of Randolph students and faculty are testing samples of antimicrobial surfaces to find out how long their self-cleaning properties last and how often they should be replaced. Chemistry professor Jesse Kern, Leo Galopin ’20, and Matthew Williams ’20 are examining the chemistry behind antimicrobial surfaces and just how effective they are at preventing the spread of bacteria.
“If you look closely at these surfaces, they look sort of like a mountain range or rolling hills,” Galopin explained. “It has peaks and valleys, and when you touch it, any microbes that you might have on your hand will latch onto the surface, sink in, and be destroyed.”
Meanwhile, Peter Sheldon, the Charles A. Dana Professor of Physics & Engineering, and Leif Kvarnes ’20 are testing the physical durability of antimicrobial surfaces. Scientifically known as technical tribology, Kvarnes is building two machines to simulate gradual wear and tear. One is a vertical piston that strikes and wears down the material at a rapid rate.
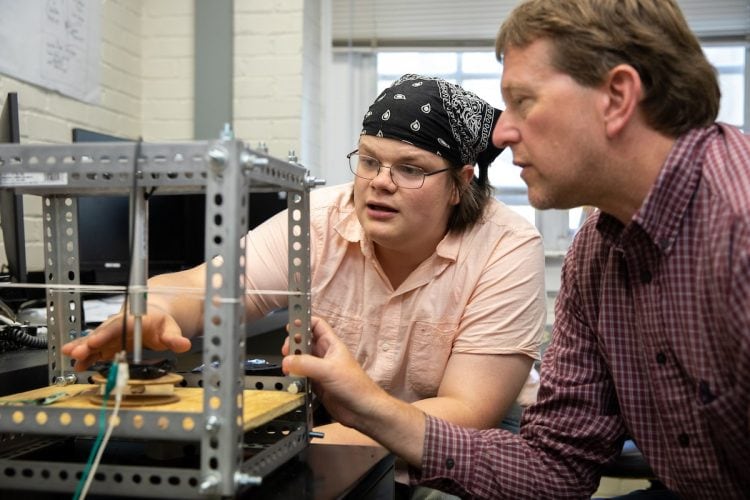
Leif Kvarnes and professor Peter Sheldon build a machine to test the durability of antimicrobial surface samples
“Imagine a cartoon piston that makes license plates on an assembly line, like the ones in Tom and Jerry,” Kvarnes joked.
The second machine he is building produces a horizontal swiping effect. He’s even coined his own terms for the process.
“We refer to a unit of wear as one ‘thwack,’” Kvarnes grinned. “One thwack equals one swipe and a return, or one bang. It’s very scientific stuff.”
For both groups, the primary goal is to test the efficacy of antimicrobial surfaces in a variety of ways by mimicking the conditions they face in the real world.
“We want to know how fast you should replace these materials, and how much wear and tear they can take before they stop being effective,” Williams said. “A lot of places that use them just sort of wait until the material starts to wear away, but they stop working way before that.”
The group is also collaborating with the Center for Advanced Engineering & Research in Lynchburg, which has conducted similar tests of its own on self-cleaning technology.
“From a commercial standpoint, companies don’t want to replace these more often than they need to, but you also don’t want to have something that looks like it’s cleaning but it’s not,” Kern said. “The whole purpose is that it’s supposed to be self-cleaning, so if it stops self-cleaning then you’re kind of deceiving your customers or whoever is interacting with these surfaces or materials.”
Galopin, who is a biology and chemistry double major, plans to continue similar lab research in graduate school.
“My interest in this is that it’s up and coming and new,” she said. “There’s still a lot that’s unknown about it. We know that it works, but we really don’t know exactly how or why it works, or why it’s so effective at killing bacteria. Currently, it’s really only being applied on paper surfaces, so thinking about how it can be used in more ways is really exciting.”
Kvarnes is also enjoying the opportunity to apply what he’s learned in the physics classroom.
“I like having the opportunity to design something from the ground up and understand all the steps that come in between,” he said. “This project involves a bit of everything and really allows me to incorporate all my skills.”
Tags: biology, chemistry, engineering, Engineering Physics, Jesse Kern, Leif Kvarnes, Leo Galopin, Matthew Williams, Peter Sheldon, physics, student faculty research, summer research, summer research 2019