Solar-powered water purification
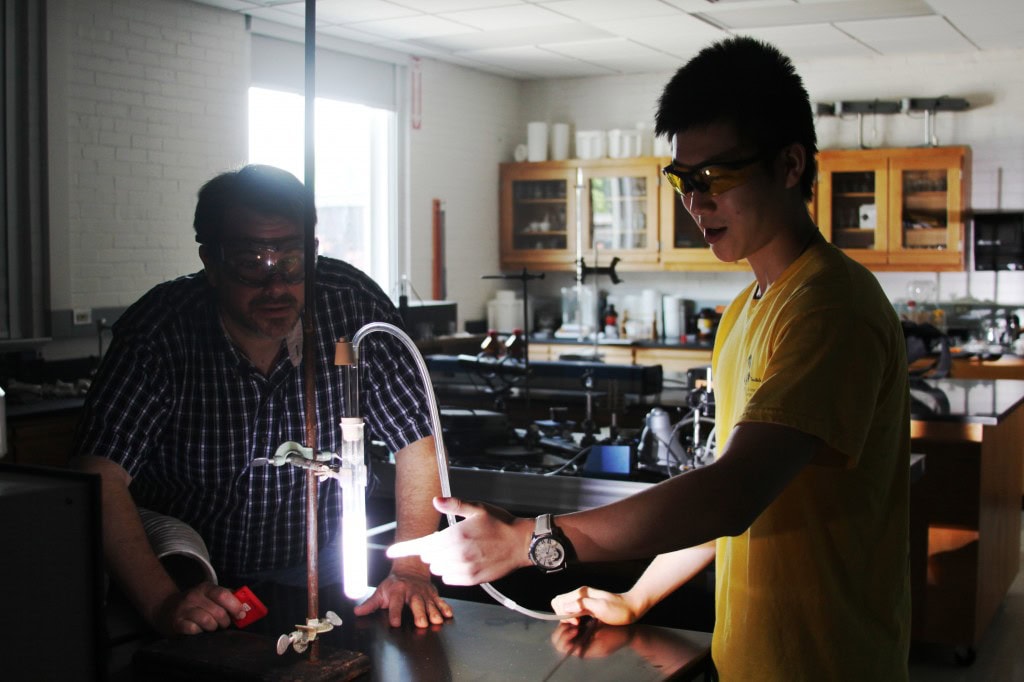
Zhe Zhang ’15 set out to learn about solar-powered water purification. But his summer research project has taught him at least as much about determination and hard work.
He has spent much of his summer trying to make one chemical reaction happen, which he originally assumed would take only a couple of days. But he takes that in stride. “It’s always fun to try different things and to finally get a solution,” he said. “I just don’t want to fail.”
Zhang has long had an interest in ways of using chemical reactions to purify water. Considering the world’s demand for potable water as well as the potential for energy shortages in the future, he thought developing a sun-powered water purification system would be important. Zhang was particularly interested in titanium dioxide, a semiconductor that is known to purify water when it is exposed to sunlight.
If only that process worked quickly.
“The process is not new, but it’s not efficient enough to be practical,” said Bill Bare, a Randolph chemistry professor advising Zhang on this research. “We’re hoping to make it a little more practical.”
Sunlight causes chemical reactions that increase energy in titanium dioxide atoms, causing the semiconductor to break down organic compounds. But it only absorbs ultraviolet light, meaning much of the light that hits it has no effect.
Bare and Zhang suggested that binding a luminescent compound called ruthenium to titanium dioxide could help increase the spectrum of light that can interact with the material. They designed an experiment to test this. Zhang began the summer by measuring the rates at which titanium dioxide would purify water on its own. But he hit a roadblock when he tried to attach the ruthenium to the semiconductor. The molecules that would make the binding possible were not connecting.
Zhang and Bare experimented with the problem for several weeks. Finally, two weeks ago they made the chemicals bind by changing the acidity of the solution, although the process needs more testing to confirm the success. Zhang looked back at the detour as an effective learning opportunity that helped him understand how to solve problems in original research.
“It’s both a process of learning and researching,” he said. “In the lab, the professor knows everything and can answer all the questions. But in research, there may be a topic that the professor is not very familiar with, and you have to research together.”
Bare added that this is part of the purpose of fostering research opportunities for students who may spend much of their lives solving research problems. “In a research project, we don’t know what’s going to work and what isn’t,” he said. “If it doesn’t work, we have to put our heads together and find out why.”
Tags: chemistry, research, summer research, summer research 2014